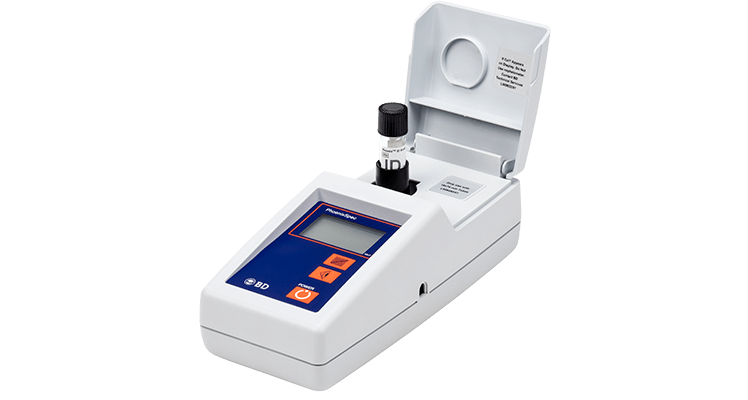
BD Phoenix™ M50 Instrument
Delivering performance, connectivity and functionality required by clinical microbiology laboratories


- Overview
- Applications
- EIFU & Resources
The BD Phoenix™ system provides on-panel doubling dilutions of antimicrobials and built-in detection resistance, including CPO, MRSA, mecA, VRSA, VRE, ESBL, HLAR, and iMLSb*.
The system requires no reagent additions post inoculation and few supplemental tests for final result determination, while utilising leak-resistant, room-temperature panel storage.
The BD EpiCenter™ data management system interfaces with the BD Phoenix™ M50 and provides intuitive, on-demand antibiogram generation and flexible reporting options.
The BD Phoenix™ M50 instrument determines growth using both an oxidation-reduction indicator and turbidity growth detection to help provide both rapid and accurate susceptibility results.
The BD Phoenix™ system utilises on-panel doubling dilutions of antimicrobial concentrations for determination of MIC results.
The BD Phoenix™ M50 instrument employs a delayed growth algorithm that may defer reporting for slow-growing organisms until additional instrument readings are collected. This feature enables an accurate determination of growth or no-growth, providing additional assurance that results are accurate.
The BD Phoenix™ system provides on-panel doubling dilutions of antimicrobials and built-in detection of resistance, including CPO, MRSA, mecA, VRSA, VRE, ESBL, HLAR, and iMLSb*.
*Depending on BD Phoenix™ panel type and organism identification.
The BD EpiCenter™ data management system offers the BDXpert system, which implements CLSI/EUCAST breakpoints and SIR interpretations. BD EpiCARE™ is an optional extension of the BD EpiCenter™ system that gives users the ability to define customised rules and actions to ensure compliance for the reporting of microbiology data that may be specific to their organisation.
The BD Phoenix™ M50 instrument offers a compact and stackable design (up to two instruments) that allows for increased testing volumes by doubling capacity within the same footprint.
The system requires minimal maintenance, no reagent additions post inoculation and few supplemental tests for final result determination, and utilises leak-resistant, room-temperature panel storage.
The BD Phoenix™ AP automated inoculation preparation instrument may help to reduce sample preparation workflow burdens, reducing total hands-on time per sample by 50% compared to manual BD Phoenix™. 1
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
*Depending on BD Phoenix™ panel type and organism identification.
Reference
- Junkins A, et al. Comparison of BD Phoenix AP Workflow with Vitek 2. J. Clin. Microbiol. 2010. 48 (5): 1929-1931. Study was supported by grants from BD Diagnostic Systems.
BD-38368
- The BD Phoenix™ M50 system instrument offers identification-only panels and combination ID/AST panels, using 51 wells for identification substrates.
- The system can provide rapid identification of most clinically significant Gram-negative and Gram- positive bacteria, as well as yeast.
- The BD Phoenix™ M50 system offers 85 wells for antimicrobial dilutions on standard AST-only panels and combination ID/AST panels, and 136 wells on the BD Phoenix™ Emerge™ panel.
- The instrument can provide susceptibility results for most clinically significant Gram-negative and Gram-positive bacteria.
- The BD Phoenix™ M50 system panels test for several resistance markers* such as** :
- Gram-positive bacteria
- HLAR—High Level Aminoglycoside Resistant Enterococcus
- iMLSb—Inducible Clindamycin Resistance
- MRSA—based on Oxacillin Interpretation with Staphylococcus species
- mecA – detection of mecA-mediated resistance in Staphylococcus aureus
- BL- Staphylococcus ß-Lactamase (Nitrocefin based test)
- VRSA—Vancomycin-Resistant Staphylococcus aureus
- VRE—based on Vancomycin interpretation
- Gram-negative bacteria
- CPO—Carbapenemase Producing Organism
- ESBL—offered on Gram-negative panels and requires no additional disc diffusion or E-Test confirmation.
- Gram-positive bacteria
* Depending on BD Phoenix™ panel type and organism identification
- The BD Phoenix™ CPO detect test, available on certain BD Phoenix™ Gram-negative panels, provides information, including Ambler classification, to help guide clinicians in their treatment decisions.
- This test is available in two-panel configurations that allow microbiology labs to test for CPOs as part of routine susceptibility testing on BD Phoenix™ panels:
- 2-well configuration—provides detection of Carbapenemase-producing organisms in Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii
- 9-well configuration—provides detection of carbapenemase-producing organisms and Ambler classification (A, B or D) in Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii
- The BD Phoenix™ CPO detect test is currently the only phenotypic test on an automated AST system that provides CPO detection and Ambler classification.
- On average, the BD Phoenix™ CPO detect test detects and classifies CPOs from isolated colonies within 6 to 11 hours.**
**BD internal studies—data on file
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
*Depending on BD Phoenix™ panel type and organism identification.
Reference
- Junkins A, et al. Comparison of BD Phoenix AP Workflow with Vitek 2. J. Clin. Microbiol. 2010. 48 (5): 1929-1931. Study was supported by grants from BD Diagnostic Systems.
BD-38368
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
*Depending on BD Phoenix™ panel type and organism identification.
Reference
- Junkins A, et al. Comparison of BD Phoenix AP Workflow with Vitek 2. J. Clin. Microbiol. 2010. 48 (5): 1929-1931. Study was supported by grants from BD Diagnostic Systems.
BD-38368