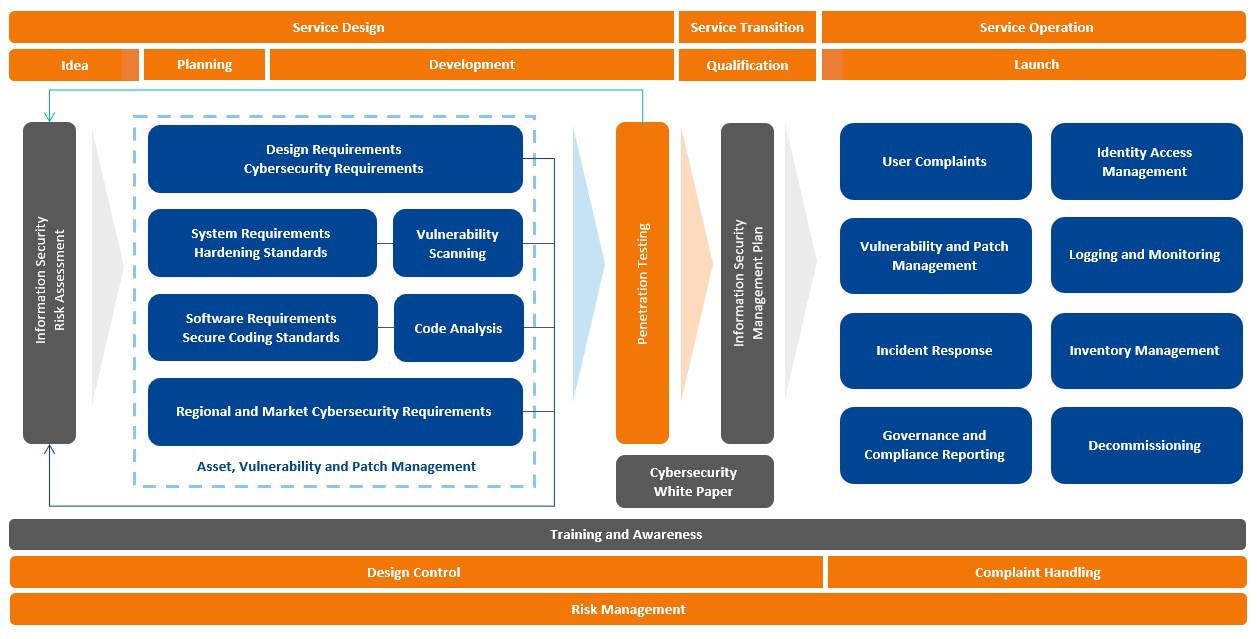
Cybersecurity is one of the most critical issues impacting the healthcare industry. At BD, we maintain an unwavering commitment to security by design, in use and through partnership. We strive to ensure our products, systems and customer environments maintain high security standards so our customers can focus on what matters most: caring for patients.
While we maintain robust security protocols, we also recognize that new security threats emerge daily, from attempts to compromise healthcare data to coordinated efforts to disrupt clinical workflows or manufacturing. We recognize that our customers cannot protect what they don’t know. That’s why we believe transparency and collaboration are essential. As we build a strong community of practice, working closely with our customers, industry regulators and security researchers, we’re improving cybersecurity and resilience across the industry.
— Rob Suárez, Vice President and Chief Information Security Officer