

- Overview
- EIFU & Resources
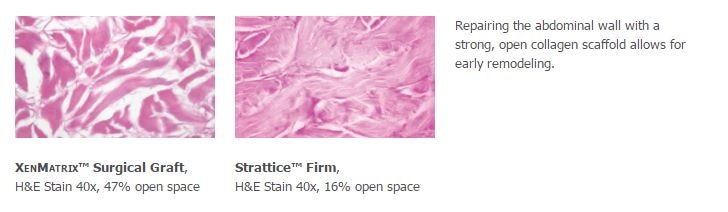
- Acellular non-crosslinked porcine collagen scaffold
- Open collagen structure allows early cellular infiltration and revascularization as demonstrated in preclinical studies1
- XenMatrix™ Surgical Graft has almost 3x more open space than Strattice™ Firm.1
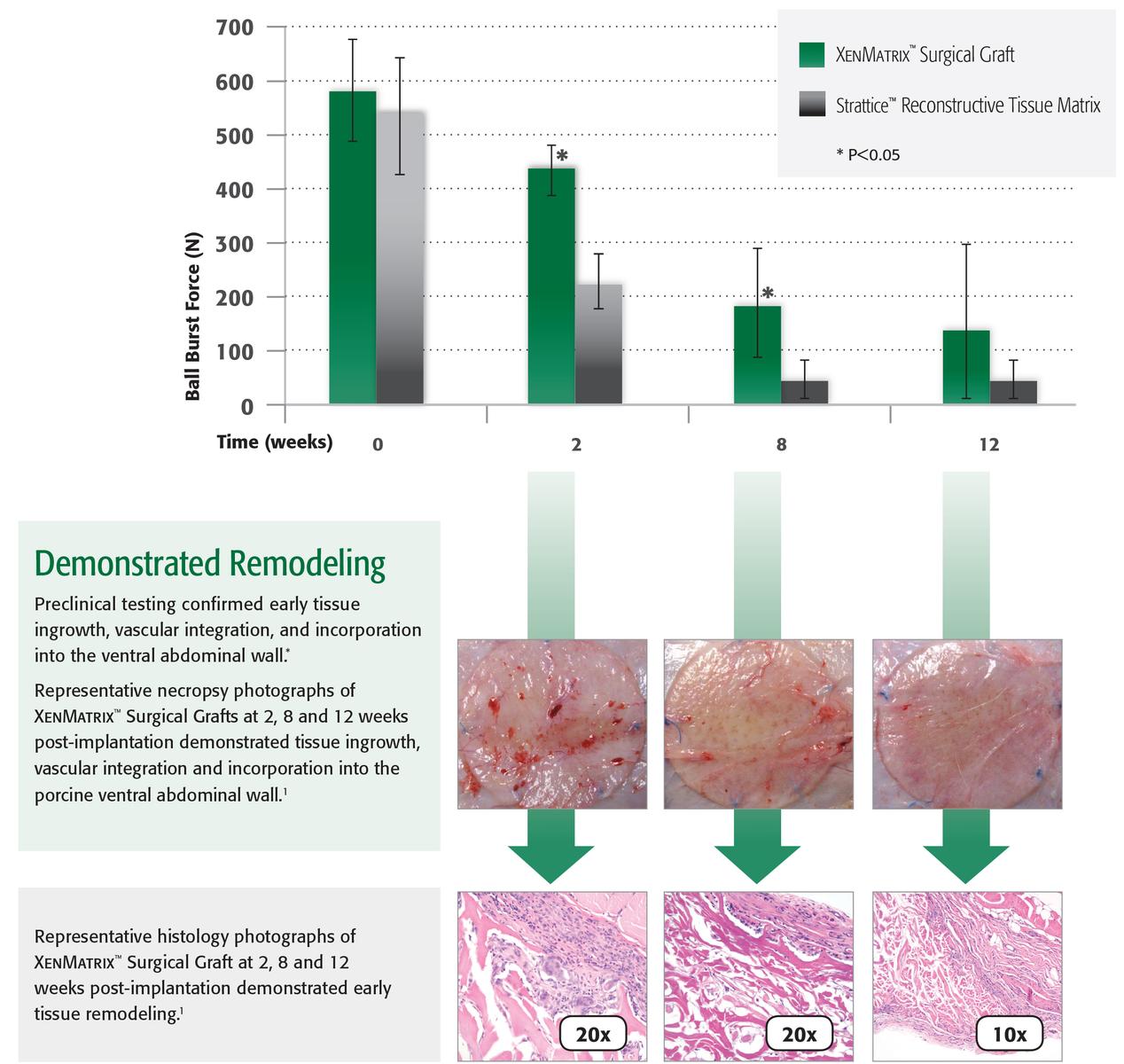
Preclinical studies demonstrated:
- Mechanical strength2
- Maintains strength during the early healing process1
- Peer reviewed clinical data2
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Preclinical data on file, results may not correlate to clinical performance
Deeken CR, Eliason B, Pichert M, Grant S, Frisella M, Matthews B. Differentiation of Biologic Scaffold Materials Through Physiomechanical, Thermal and Enzymatic Degradation Techniques. Ann Surg 2012. Mar; 255(3):595-604.
Disclaimers
Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
Indications
XenMatrix™ Surgical Graft is intended for implantation to reinforce soft tissue defects where weakness exists in abdominal and incisional hernias.
Contraindications
XenMatrix™ Surgical Graft should not be used on patients with known sensitivity to porcine products.
Not for reconstruction of cardiovascular defects.
Not for reconstruction of central nervous system or peripheral nervous system defects.
Use of this product in applications other than those indicated has the potential for serious complications.
Warnings
This device has been designed for single use only. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness or death of the patient or end user.
DO NOT RESTERILIZE
Prior to use, carefully examine package and product to verify neither is damaged and that all seals are intact. Do not use if the package is damaged or open, or if the center of the temperature indicator on the foil pouch is black.
If an infection develops, it should be treated aggressively.
An allergic reaction, which is unrelated to other therapy, is an indication to consider removal of XenMatrix™ Surgical Graft.
After use, any unused product and packaging should be treated as a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable local, state, and federal laws and regulations.
The safety and effectiveness of XenMatrix™ Surgical Graft in the following applications has not been evaluated or established: a. Pregnant or breastfeeding women b. Pediatric use.
Precautions
Please read all instructions prior to use.
Only physicians qualified in the appropriate surgical techniques should use this surgical graft.
Strict aseptic technique should be followed.
U. S. federal law restricts this device to sale by or on the order of a physician.
The surgeon should thoroughly understand the surgical procedure and the performance characteristics of the surgical graft.
Place the graft in maximum possible contact with healthy, well-vascularized tissue to promote cell ingrowth and tissue remodeling.
When unable to close the skin over the XenMatrix™ Surgical Graft, ensure that the implant remains moist. Avoid drying of the implant through “continued suction devices” as this may negatively impact the performance of the implant.
Adverse Reactions
Possible complications may include but are not limited to allergy, seroma, infection, inflammation, adhesion, fistula formation, hematoma, and recurrence of the tissue defect. Please consult product package insert for more detailed safety information and instructions for use.
BD-51650 (10/22)