Ventrio™ ST Hernia Patch
Self-Expanding Bioresorbable Coated Permanent Mesh for Soft Tissue Reconstruction with SorbaFlex™ Memory Technology.


- Overview
- EIFU & Resources
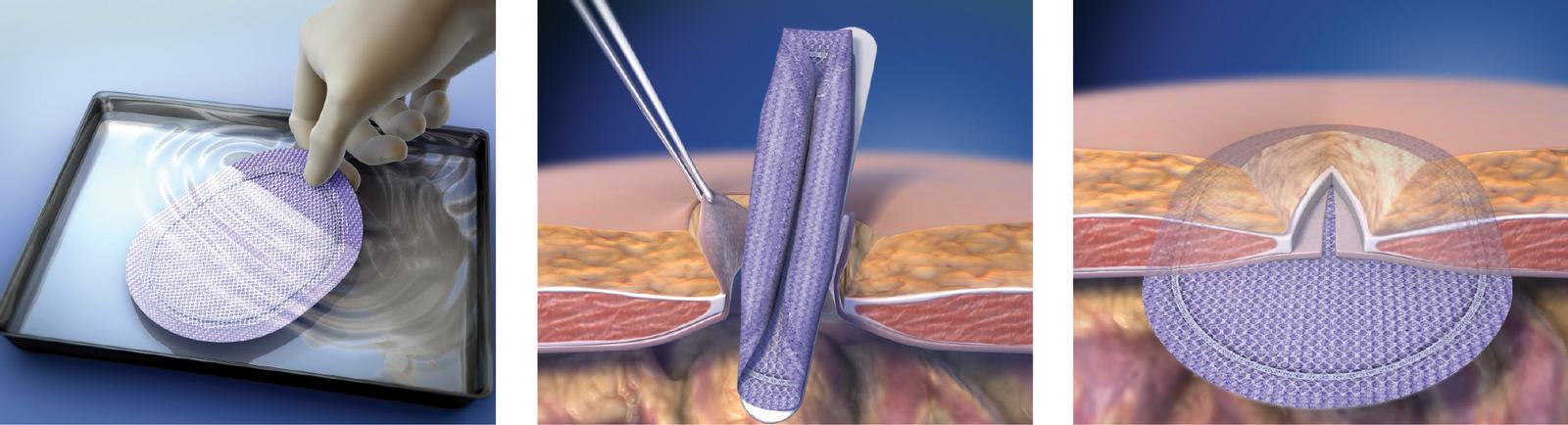
Easy
- Provides the benefits of a laparoscopic repair through the ease of a smaller incision.
- SorbaFlex™ Memory Technology allows the patch to “spring open,” lay flat to maintain shape and then fully absorbs over time.1
- Simplifies placement and positioning of the patch throughout the ventral hernia repair.
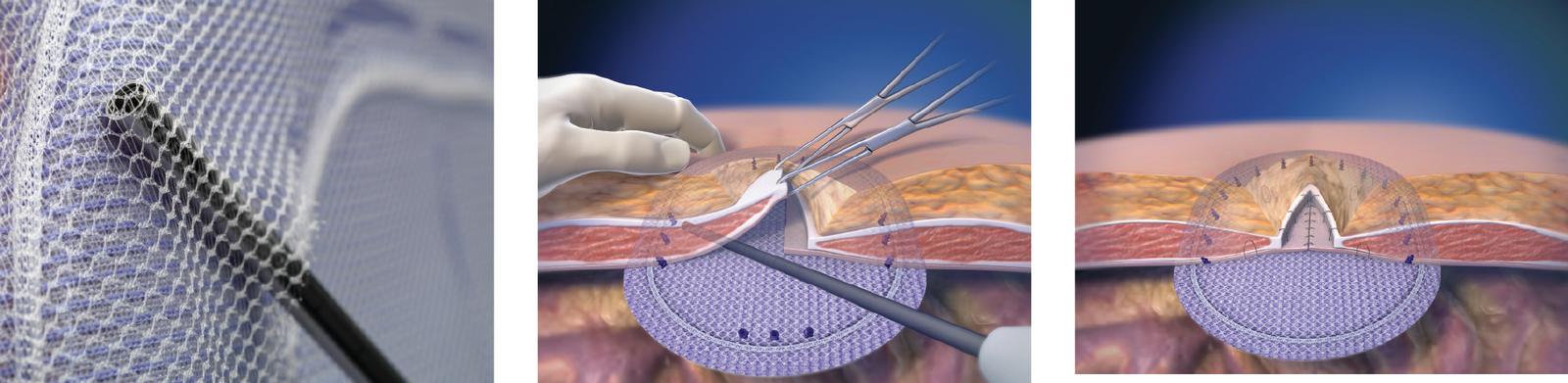
Efficient
- Unique pocket aids in the proper placement and positioning of the patch.
- Designed to facilitate the use of mechanical fixation devices and/or sutures.
- Available in a variety of shapes and sizes to accommodate defect sizes and locations.
Proven1
- Hydrogel barrier is based on Sepra® Technology.
- Uncoated monofilament polypropylene mesh allows for complete tissue ingrowth leading to a strong repair.
- Materials have been used in general surgery for years with demonstrated clinical success.2
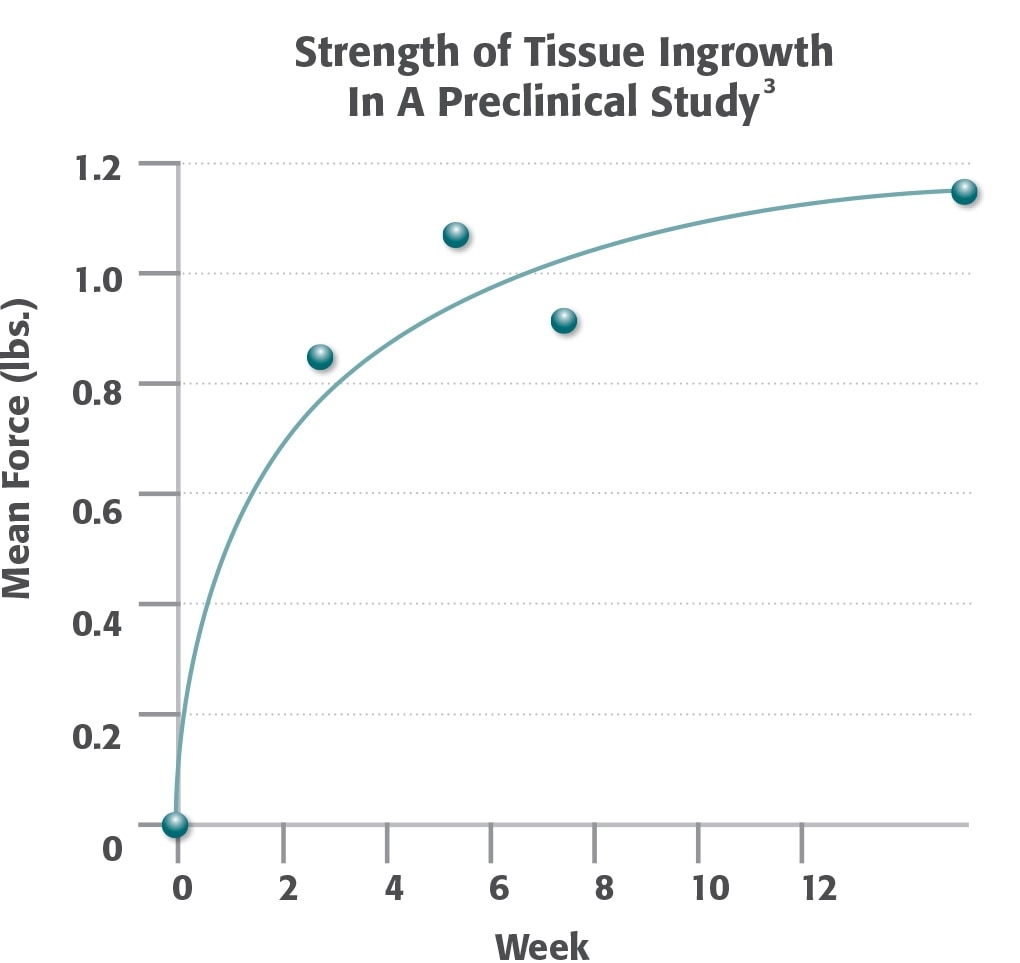
Logarithmic regression curve of mean force of lap-shear strength as a function of time. 74% of the 12 week strength is achieved by 2 weeks post-operatively.

Ventrio™ ST Hernia Patch Preclinical Results
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Indications
The Ventrio™ ST Hernia Patch is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair of ventral, incisional, and umbilical hernias.
Contraindications
- Do not use this mesh in infants, children, or pregnant women, whereby future growth may be compromised by the use of such mesh materials.
- The use of this mesh has not been studied in breastfeeding or pregnant women.
- Do not use this mesh for the reconstruction of cardiovascular defects.
- Literature reports that there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
Warnings
- The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require the removal of the mesh.
- If the unused mesh has been in contact with instruments or supplies used on a patient or contaminated with bodily fluids, discard with care to prevent risk of transmission of viral infections.
- To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
- This mesh is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
- This mesh has been designed for single use only. Reuse, resterilization, reprocessing and/or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the mesh and may lead to mesh failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the mesh and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the mesh may lead to injury, illness, or death of the patient or end user.
- This mesh should be used once the exterior foil pouch has been opened. Do not store for later use. Unused portions of the mesh should be discarded. Ensure proper orientation; the bioresorbable coated side of the mesh should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the mesh is placed in direct contact with the bowel or viscera.
- Do not cut or reshape the Ventrio™ ST Hernia Patch, as this could impact its effectiveness. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament duringinsertion or fixation. If the SorbaFlex™ PDO monofilament is cut or damaged, additional complications may include but are not limited to, bowel or skin perforation and infection.
- Follow proper folding techniques for all patches as described in these Instructions for Use as other folding techniques may compromise the SorbaFlex™ PDO monofilament (Figure 2).
- To ensure a strong repair, the mesh should be secured with tacks or sutures through the polypropylene mesh structure or full mesh. Suturing or tacking on the edge of the mesh alone is not recommended.
- This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
- This mesh is not for the use of treatment of stress urinary incontinence.
Adverse Reactions
Possible complications include, but are not limited to, seroma, adhesions, hematomas, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.
References
- Preclinical data on file at C. R. Bard. Results may not correlate to performance in humans.
- Iannitti, D. et. al. “Technique and Outcomes of Abdominal Incisional Hernia Repair Using a Synthetic Composite Mesh: A Report of 455 cases.” Journal of the American College of Surgeons. 2008 Jan; 206 (1):83-8.
- Majercik, S. et al. “Strength in tissue attachment to mesh after ventral hernia repair with synthetic composite mesh in a porcine model.” Surg Endosc (2006) 20: 1671-1674.
BD-61476