Progel Platinum™ Surgical Sealant
A unique option designed to address postoperative air leak complications.


- Overview
- EIFU & Resources

Designed
PEG: strength and flexibility
The proprietary polyethylene glycol (PEG) used in the Progel PlatunumTM formulation is a non-toxic, non immunogenic molecule9 which lends the hydrogel its ability to stretch.
rHA: optimal contact and adherence
Recombinant human albumin (rHA) is a large protein molecule that provides Progel PlatunumTM with its adhesive strength.
Designed
- The only sealant specially designed for the lung and its unique characteristics
- Combines Polyethylene Glycol (PEG) and Recombinant human albumin (rHA) to form a flexible hydrogel
- Gels at the tissue site, binding directly to the lung for optimal adherence and an airtight seal
- Strong enough to withstand re-expansion of the lung within 2 minutes of application1
- Highly elastic to allow the lung to expand and contract naturally during respiration
- The patented Progel Platinum™ Extended Tip allows for customized application from a single, easy-to-use device
Indicated
- Progel Platinum™ Surgical Sealant is the only sealant approved specifically to seal pleural air leaks in Europe.
- Progel Platinum™ forms a highly-flexible hydrogel specifically designed for use on the lung.
- Progel™ Pleural Surgical Sealant (PALS) is the only product FDA approved to treat air leaks regardless of your approach. 2
Clinically Proven
- The only sealant clinically proven to effectively treat air leak complications in both open and minimally invasive thoracic surgery1
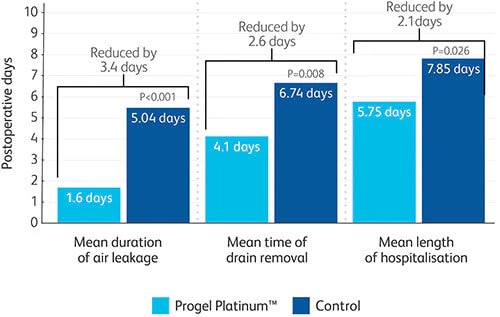
- Clinically proven to seal leaks and reduce length of hospital stay by 2.1 days in a prospective, randomized, multi-center trial.8
Cost Effective
- As many as 58% of lung surgery patients will have an air leak in the OR3
- Air leaks are the most important determinant of hospital length of stay (LOS), and a prolonged air leak can extend length of stay by more than 4 days.5
- The total cost of treating prolonged postoperative air leaks and their associated clinical complications is substantial.6
- The total 90-day postoperative cost of a prolonged air leak is estimated to be an additional 5000€ per patient.7
- Progel Platinum™ Surgical Sealant has been shown to effectively seal air leaks during lung surgery, reducing length of hospitalization by 2.1 mean days, potentially minimising associated complications and cost-of-care.8
Progel Platinum™ MOA animation
Progel Platinum™ Surgical Sealant
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative
References
Progel Platinum™ Surgical Sealant Instructions for Use. Data on file.
Progel™ Pleural Surgical Sealant (PALS) is not available in EU, its only difference with Progel Platinum™ is that the latter is comprised of a proprietary combination of Recombinant human albumin (rHA) while PALS has human serum albumin (HSA) as the main component.
Allen, Mark S. et al, “Prospective Randomized Study Evaluating a Biodegradable Polymeric Sealant for Sealing Intraoperative Air Leaks That Occur During Pulmonary Resection” Annals of Thoracic Surgery 2004; 77:1792-1801. Pivotal study. Data on file.
Brunelli et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg 2004; 77: 1205-1210. Based on the reported incidence of prolonged postoperative air leak. Data on file.
Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis. 2014 Mar;6(3):271-84. Data on file.
Varela G. et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg. 2005 Feb;27(2):329-33.
Brunelli A et al. Ninety-day hospital costs associated with prolonged air leak following lung resection. Interact Cardiovasc Thorac Surg. 2020 Oct 1;31(4):507-512.
Zaraca F. et al. Cost-effectiveness analysis of sealant impact in management of moderate intraoperative alveolar air leaks during video-assisted thoracoscopic surgery lobectomy: a multicentre randomised controlled trial. J Thorac Dis. 2017 Dec;9(12):5230-5238)
Davol, Inc. Comprehensive Biocompatibility Study. Data on file.
Disclaimers
Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
Indications
Progel Platinum™ Surgical Sealant is a single use device intended for application to visible air leaks on the visceral pleura after standard visceral pleural closure techniques have been employed during resection of lung parenchyma.
Contraindications
Do not use Progel Platinum™ in patients who have a history of an allergic reaction to rice, rHA or other device components.
Do not use Progel Platinum™ in patients who may have insufficient renal capacity for clearance of the Progel Platinum™ polyethylene glycol load.
Do not apply Progel Platinum™ on open or closed defects of main stem or lobar bronchi due to a possible increase in the incidence of broncho-pleural fistulae, including patients undergoing pneumonectomy, any sleeve resection or bronchoplasty.
Do not apply Progel Platinum™ on oxidized regenerated cellulose, absorbable gelatin sponges or any other surface other than visceral pleura as adherence and intended outcome may be compromised.
Warnings
Progel Platinum™ should be used only as described in these instructions for use.
Progel Platinum™ should be stored between 2°C to 8°C (36°F to 46°F) at all times. Do not freeze. Failure to store Progel Platinum™ within the recommended temperature range may result in poor product performance.
Inspect the packages before opening. Do not use Progel Platinum™ after the “expiration”date, because sterility or performance may be compromised. If package and/or product integrity have been compromised (i.e., damaged package seal, or broken glass), do not use or resterilize the contents.
This device is packaged and sterilized for single use only.
Do not reuse or reprocess. Reuse or reprocessing may compromise the structural integrity of the device, and/or lead to device failure that in turn may result in patient injury, illness or death. Also, reprocessing of single use devices may create a risk of contamination and/or patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient. Refer to additional ‘precautions’ in this IFU.
Do not use more than 30 mL of Progel Platinum™ per patient.
This device contains the following substance defined as CMR 1B in a concentration above 0.1% weight by weight: Cobalt; CAS No. 7440-48-4; EC No. 231-158-0. Current scientific evidence supports that medical devices manufactured from stainless-steel alloys containing cobalt do not cause an increased risk of cancer or adverse reproductive effects. For more information, please consult the ECHA website: .https://echa.europa.eu/home.
Precautions
The safety and effectiveness of Progel Platinum™ has not been established in patients with the following conditions:
Less than 18 years of age, pregnant or nursing women.
Contaminated or dirty pulmonary resection cases.
The presence of an active infection.
In the presence of other sealants, hemostatic devices or products other than sutures and staples used in standard visceral pleural closure.
Visceral pleural air leak due to spontaneous pneumothorax, any non-resective pulmonary tissue trauma, or malignancy as well as congenital or acquired functional or anatomic defect.
In any area or tissue other than the visceral pleural surface as indicated.
Progel Platinum™ use with any additive (e.g. antibiotics) to any component has not been studied.
Inspect sterile package and seal prior to use. Do not use if sterile package or seal are damaged or open.
Do not use rehydrated crosslinker after 20 minutes, as the performance of Progel Platinum™ may be compromised.
Interruption of the application for approximately 10 seconds may result in occlusion of the spray tip. If occlusion occurs, remove the spray tip, wipe the end of the applicator to remove any fluid, and attach a new spray tip (provided) onto the end of the applicator.
Progel Platinum™ is intended for single use only. Do not re-sterilize or reuse any component. The re-sterilization or reuse of Progel Platinum™ may result in the loss of functional product performance.
During Progel Platinum™ application, if possible, target lung ventilation should be stopped to reduce air leakage from the targeted sites and to minimize tissue movement during Progel Platinum™ application. If the patient needs target lung ventilation, a reduced tidal volume is recommended.
During preparation and between sprays, wipe the applicator tip with clean, sterile gauze to remove any residual liquid that may have been expressed. Avoid mixing of components: do not wipe from one cartridge opening across to the other – wipe each opening separately.
The unique design of the spray tip allows for Progel Platinum™ application as a spray or as a stream (firm steady pressure on the push rod will yield a spray, while gentle pressure will yield a stream).
During Progel Platinum™ application, keep the applicator tip approximately 5 cm (2 in) away from target area to avoid creating bubbles in Progel Platinum™ material during application. Bubbles may compromise the adherence and/ or mechanical properties of Progel Platinum™.
Discard unused material in accordance with standard practice for Progel Platinum™ components.
Recombinant Human Albumin - rHA in the Progel Platinum™ kit is derived from rice and does not contain any human or animal source. The rHA is equivalent to human serum albumin, without the human blood origin.
During applicator assembly, the Progel Platinum™ push rod is designed to lock into the applicator housing. Forced removal of the locking push rod from the applicator housing may result in potential damage to the applicator system or the chemistry cartridges.
Adverse reactions
As with medical devices used in a surgical procedure, adverse reactions are always possible. The following lists possible adverse reactions associated with lung surgeries:
Fever Infection
Atrial Fibrillation Renal Function Abnormal
Nausea Hypertension
Pneumothorax Emphysema
Anemia Pain
Death Pneumonia
Heart Problems Embolism
Pleural Effusion Urinary Problems
Renal Failure, Acute Respiratory Failure
Arrhythmia Flu-like Symptoms
Bleeding Hemorrhage
Heart Failure Respiratory Insufficiency
Edema Site Hematoma
Allergic Reaction Gastroesophageal Reflux
Dyspnea Respiratory Distress
BD-54055 (02/22)