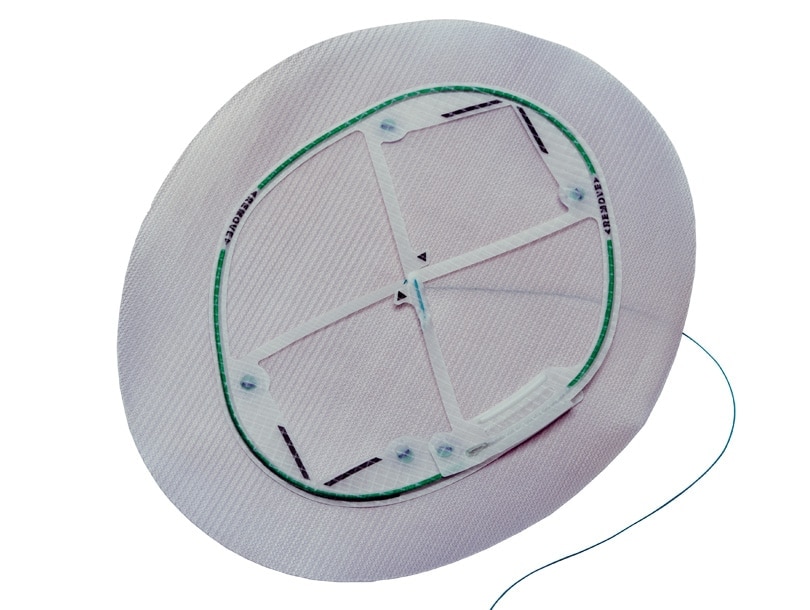
Echo PS™ Positioning System with Ventralight™ ST Mesh
Low profile bioresorbable coated permanent mesh with positioning system designed for laparoscopic ventral hernia repair


- Overview
- EIFU & Resources

Mesh Preparation
- Comes pre-attached with Ventralight™ ST Mesh.
- Designed to ensure that barrier protection is oriented towards viscera.
- Reduces mesh preparation time and user frustration.1
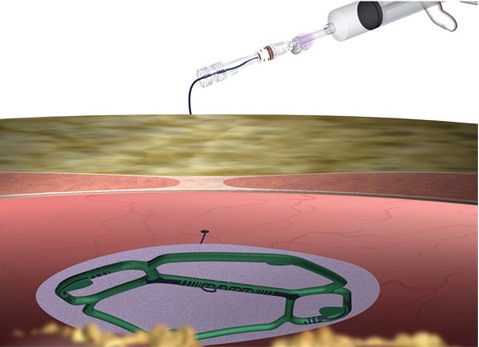
Deployment
- Does not require dedicated trocar.
- Potentially reduces patient trauma.
- Following mesh introduction, trocar is free to be used with other laparoscopic instruments.
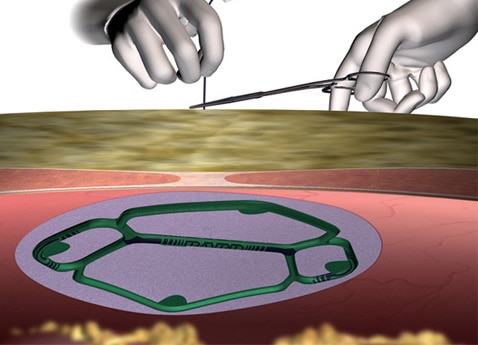
Positioning
- Technique designed to center mesh over defect.
- Alleviates guesswork and facilitates centering.
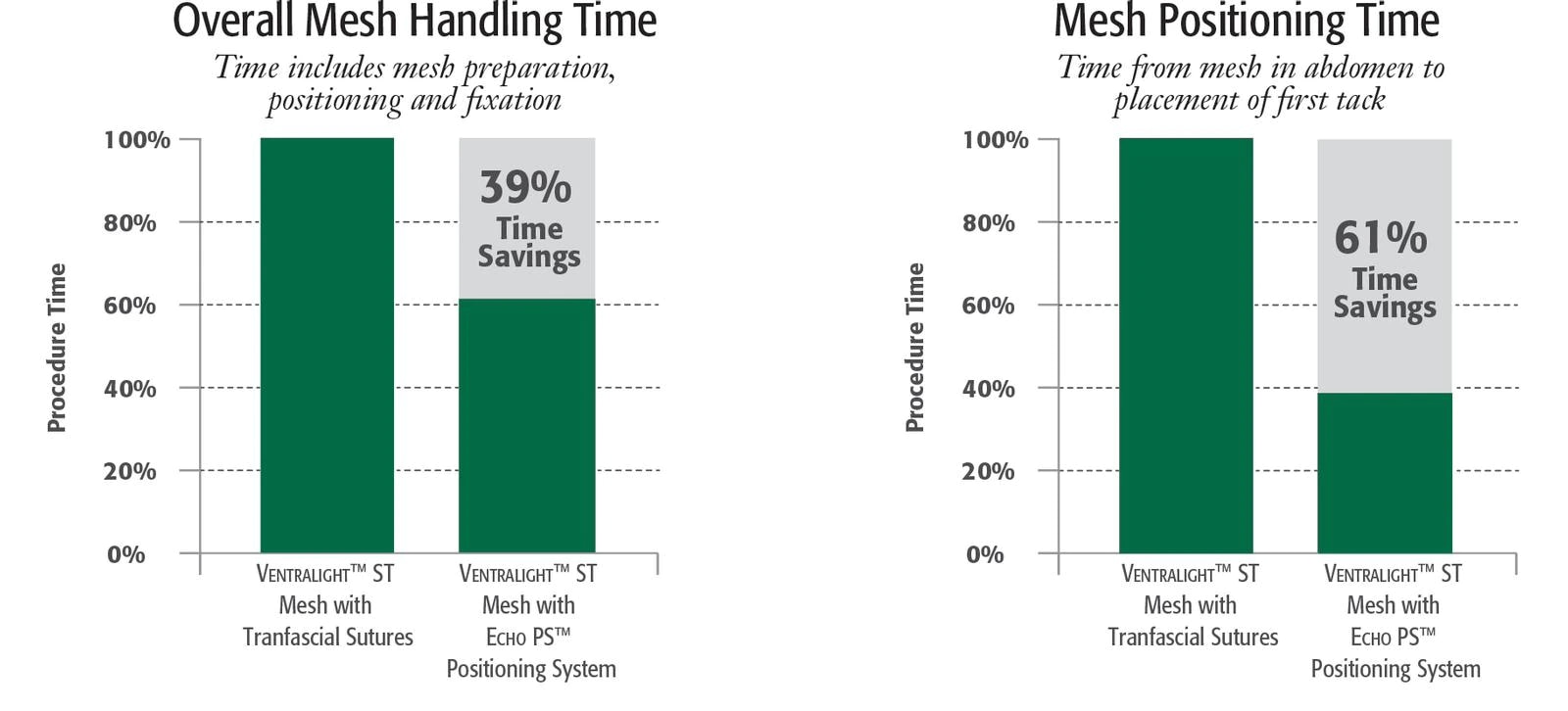
Compared to positioning with transfascial sutures, Echo PS™ Positioning System:
- Reduces mesh positioning by over 60%1
- Reduces procedure time variability by 84%1
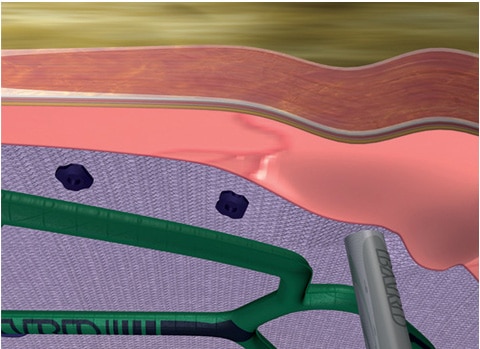
Fixation
- Requires only one set of hands.
- Once positioned with the Echo PS™ Positioning System, mesh stays in place allowing the surgeon to use both hands for fixation.
- No assistance or handoff needed to hold mesh in place during fixation potentially leading to increased accuracy.
- Unique flexible balloon design.
- Allows mesh to conform to the abdominal wall.
- Occupies less intraabdominal space and is designed not get in the way of fixation.
Reproducible results
The Echo PS™ Positioning System puts the control into the surgeon’s hands resulting in reproducible results in less time.1
- The Echo PS™ Positioning System technique eliminates the guesswork by facilitating accurate mesh centering and assisted fixation without the need for an extra set of hands.
- More than 3 years of clinical experience and a growing number of surgeons adopting the technology into their standard practice.
- Award-winning innovative design streamlines laparoscopic procedures, saving time and reducing procedure time variability by 84%.1
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
When compared to positioning with transfascial sutures in a preclinical study.
Date on file at C. R. Bard, Inc. Results may not correlate to performance in humans.
Disclaimers
Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
Indications
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, in the repair of ventral, incisional, and umbilical hernias. The Echo PS™ Positioning System is intended to be used to facilitate the delivery of the Ventralight™ ST Mesh during laparoscopic hernia repair.
Contraindications
Do not use this mesh in infants, children, or pregnant women, whereby future growth may be compromised by the use of such mesh materials.
The use of this mesh has not been studied in breastfeeding or pregnant women.
Do not use this mesh for the reconstruction of cardiovascular defects.
Literature reports there is a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
Warnings
The use of any permanent mesh in a contaminated or infected wound could lead to infection, fistula formation, and/or extrusion of the mesh.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
If unused mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard with care to prevent risk of transmission of viral infections.
To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
This device is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
This device has been designed for single use only. Reuse, resterilization, reprocessing and/or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient or end user.
The mesh should be used once the exterior foil pouch has been opened. Do not store for later use. Unused portions of the mesh should be discarded.
Ensure proper orientation; the coated side of the mesh should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera (reference Surface Orientation section).
Do not apply sharp, heat emitting, or ultrasonic tools (such as scissors, needles, tackers, diathermic tools, etc.) to the Echo PS™ Positioning System.
The Echo PS™ Positioning System should not be used with any other hernia mesh aside from those with which it comes pre-attached/packaged.
Ventralight™ ST Mesh is the only permanent implant component of the device. The inflation adapter and syringe are to be kept external to the patient and discarded after use. The Echo PS™ Positioning System (including the balloon, all connectors, and inflation tube) must be removed from the patient and appropriately discarded. It is not part of the permanent implant.
Discard Introducer Tool and all components of the Echo PS™ Positioning System (including the inflation adapter and syringe) after use. This product may be a potential biohazard. Handle and dispose in accordance with accepted medical practice and applicable local, state, and federal laws and regulations.
This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
This mesh is not for the use of treatment of stress urinary incontinence.
Precautions
Please read all instructions prior to use.
Only physicians qualified in the appropriate surgical techniques should use this device.
The safety and effectiveness of Ventralight™ ST Mesh with Echo PS™ Positioning System has not been evaluated in clinical studies for the presence of malignancies in the abdominopelvic cavity.
Visualization must be maintained throughout the course of the entire procedure. Additionally, laparoscopic removal of the Echo PS™ Positioning System must be performed under sufficient visualization of the entire device and surrounding anatomy to ensure proper removal.
Do not trim the mesh. This will affect the interface between the mesh and positioning system.
Adverse Reactions
Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction, and recurrence of the hernia or soft tissue defect.
BD-69622 (09/22)