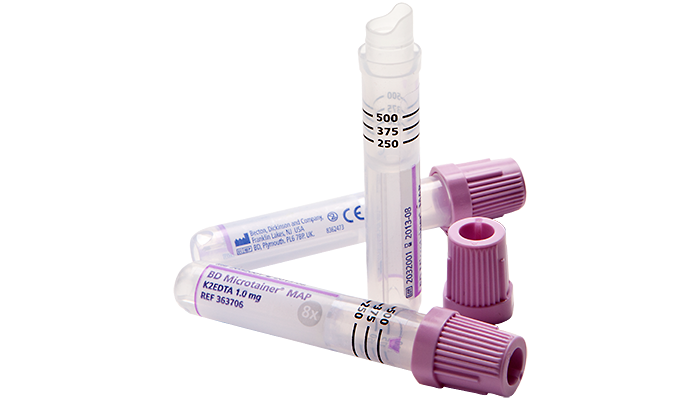
The BD Microtainer® MAP Microtube for Automated Process is the first one–piece, instrument–compatible microtube to offer both standard full–size patient identification labels as well as compatibility with most automated haematology instruments.
BD Microtainer® MAP Microtube for Automated Process
Automate processing with the first one-piece, instrument–compatible microtube

- Overview
- EIFU & Resources
The microtube streamlines operations by allowing the automated processing of your microcollection haematology samples.
It is approved for haematology testing.
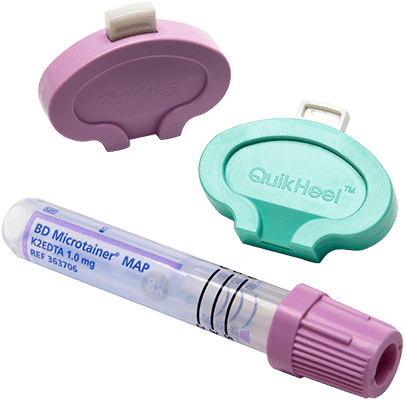
Easy to open with twist-locking mechanism that ensures no leakage.
Compatible with main haematology analysers without the need for a tube adapter.
A capillary blood tube with standard blood collection tube dimensions (13 x 75 mm) and penetrable closure.
Three clearly visible fill markings ensure the correct sample volume (250-500 μl).
Identification
A standard label can be attached directly to the sample, minimising the risk of misidentification due to missing or incomplete labelling.
Colour marking
Colour marking for identification of the type of sample.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
The Microtainer® Blood Collection Tubes are in vitro diagnostic medical devices and are CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC
The lancets are medical devices, they are CE marked in compliance with European Medical Device Directive 93/42/EEC and are CE certified by NSAI - National Standards Authority of Ireland (Notified Body Number = 0050)
BD-18635


