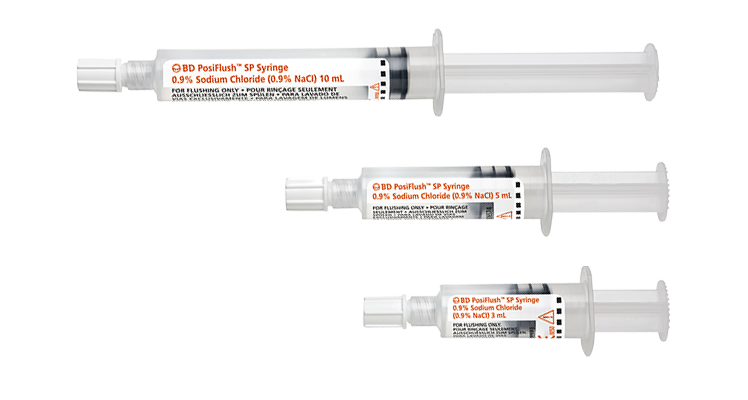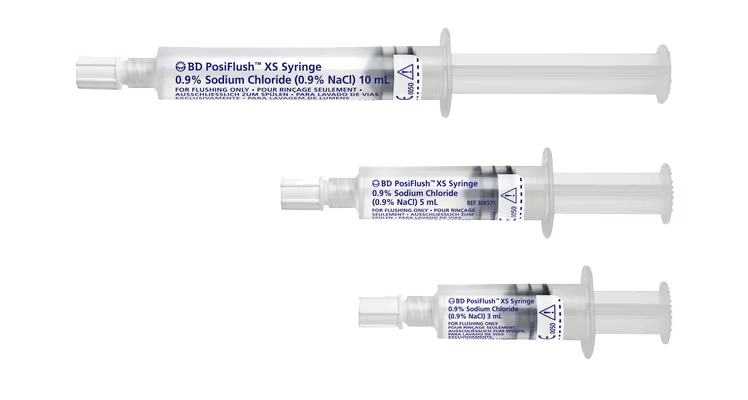


BD offers a portfolio of saline pre-filled products to meet your clinical needs, including externally sterile packaged syringes for sterile field applications. The BD PosiFlush™ portfolio of pre-filled syringes provides reliable1,2, cost-effective alternatives compared to vial-based flushing.1,3 Furthermore, they are specifically designed to help reduce the risk of medication errors4,5, may help lower the risk of catheter damage1 and reduce disposal waste.
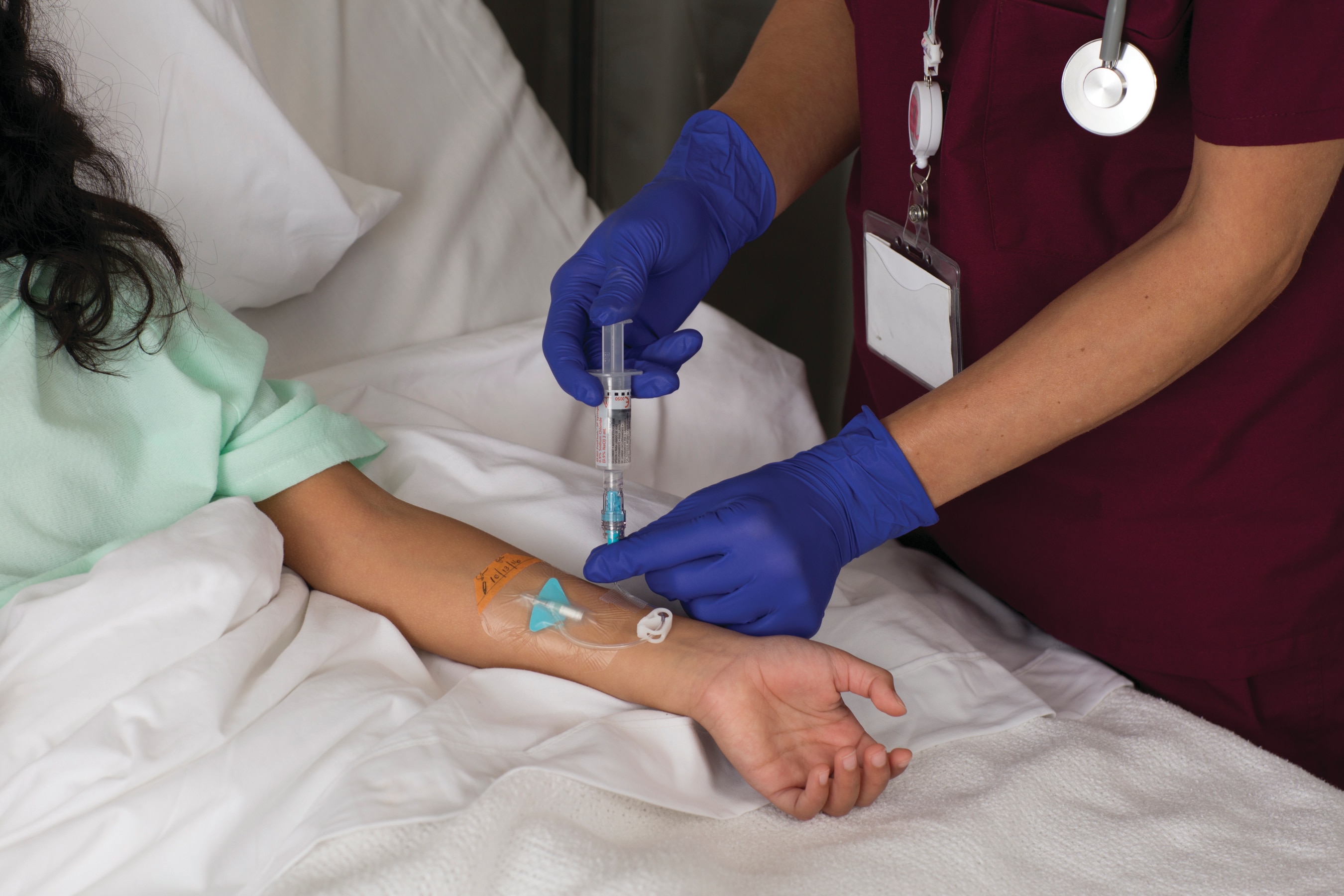
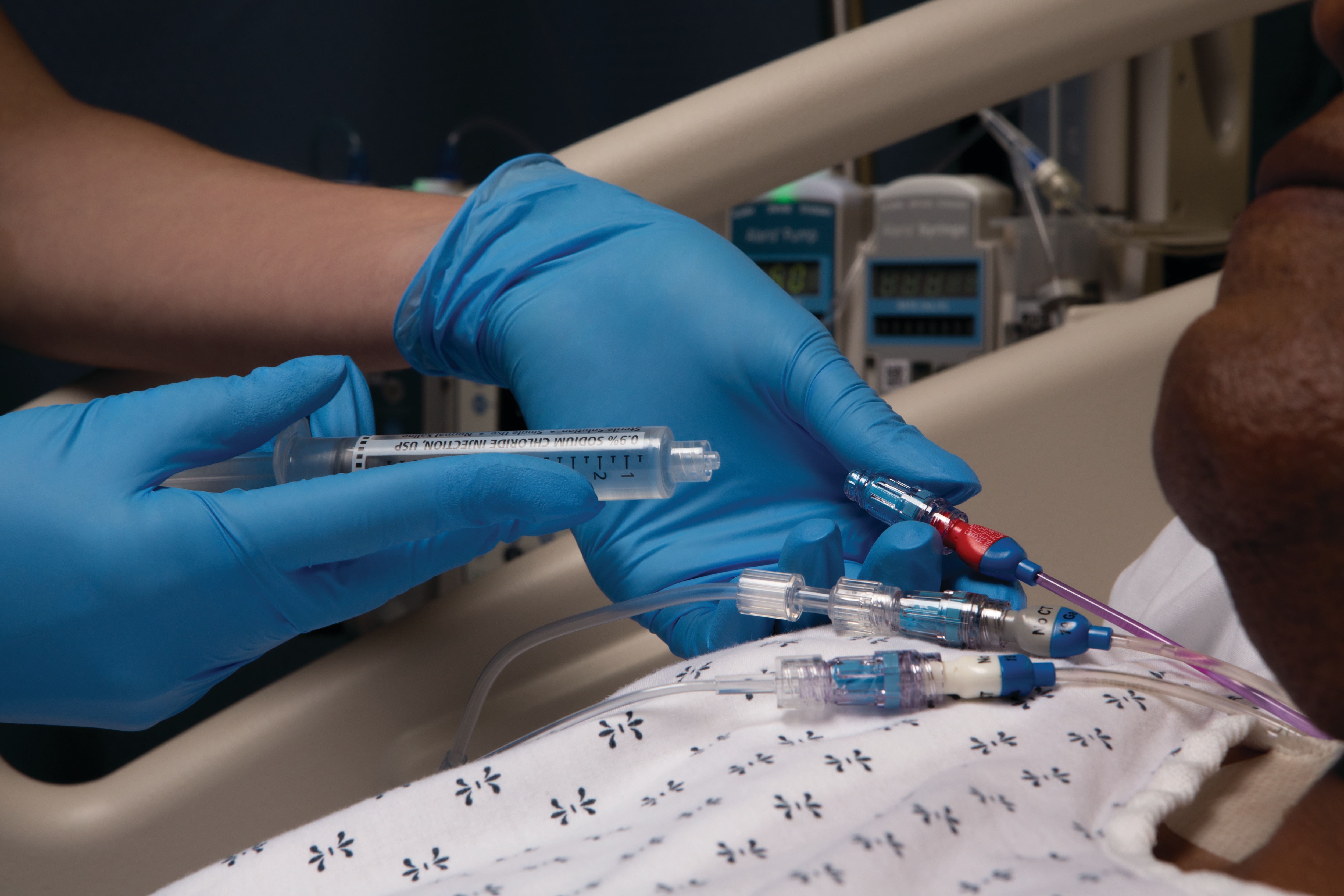
The BD PosiFlush™ Pre-Filled Syringe supports efforts to reduce the risk of medication errors by meeting Joint Commission4 and ISMP5 (Institute for Safe Medication Practices) medication labelling guidelines. It may reduce the risk of catheter-related bloodstream infection (CRBSI) when compared to manually prepared flush syringes.3
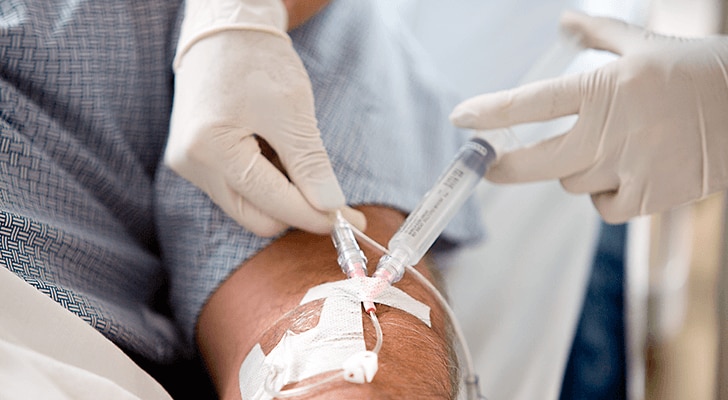
Both types, sterile fluid path syringes and externally sterile packaged syringes, come in three volumes (3 mL, 5 mL and 10 mL) of equal diameter (10 mL syringe). Because the BD PosiFlush™ 3-mL Syringe has the same diameter as a standard 10-mL syringe, it generates less pressure than smaller diameter syringes and reduces the risk of catheter damage.1
BD PosiFlush™ Prefilled Saline Syringes are sterilised using moist heat, which eliminates microorganisms.6 Tests are performed at the end of each production cycle to ensure each syringe is ready to be used for effective patient care.
Not all products and services may be available in your area. Please contact a BD representative for more information.
References
- Start K. Prefilled saline flushes. Hospital Pharmacy Europe. Published on 18 August 2010. Accessed on 24 January 2023, at https://hospitalpharmacyeurope.com/news/editors-pick/prefilled-saline-flushes/
- Medical Device Education and Training (MDET). Pre-filled syringe for flushing IV cannula; To be prescribed or not prescribed? – that is the question. NAMDET. 2020;3(1) 13-21.
- Bertoglio S, Rezzo R, Merlo FD, et al. Pre-filled normal saline syringes to reduce totally implantable venous access device-associated bloodstream infection: a single institution pilot study. J Hosp Infect. 2013;84(1):85-88.
- Joint Commission. National Patient Safety Goals Effective January 1, 2014: Hospital Accreditation Program. 2014.
- ISMP Safe Practice Guidelines for Adult IV Push Medications. ISMP. 2015.
- Brown K, Graf LM, Guyader MJ, et al. Technical Report No. 48 Moist Heat Sterilizer Systems: Design, Commissioning, Operation, Qualification and Maintenance. Bethesda, Maryland, U nited States: Parenteral Drug Association; 2010.
BD-79197 (May 2023)